ISO 13485 (THE QUALITY MANAGEMENT SYSTEMS STANDARD FOR THE MEDICAL DEVICE INDUSTRY)
Everywhere in the world, strict rules apply to medical devices and to companies concerned with their development, manufacture, sales and reconditioning. With its long years of experience as a certification body and notified body, TÜV NORD offers comprehensive service from one source - for safe access to national and international markets.
EN ISO 13485, as a quality management system for medical devices, describes requirements for regulatory purposes and describes the development, implementation and maintenance of a quality management system for medical device manufacturers and suppliers.
The standard contains detailed requirements for a quality management system which fulfils both customer requirements and the requirements for all life cycle stages of a medical device, including its components and the original raw materials used in manufacture, along with any related services.
Benefits of EN ISO 13485 certification
- Sustainable quality assurance
- Identification of potential cost savings and improvements
- High customer satisfaction
- Image improvement
- Minimization of risks
- Improved economic efficiency through process improvement
- Greater competitiveness
- Fulfilment of specific customer requirements
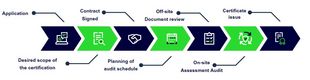
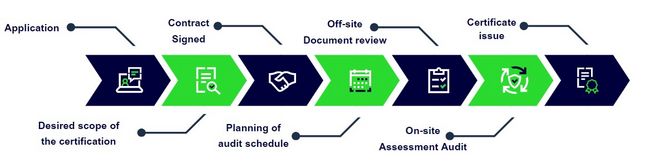
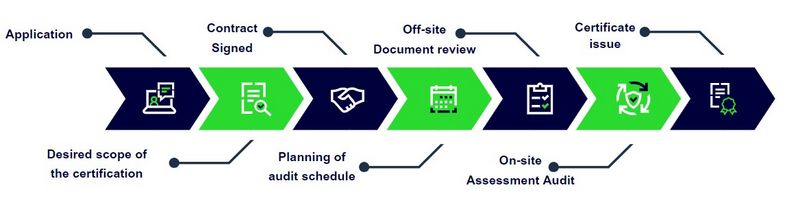
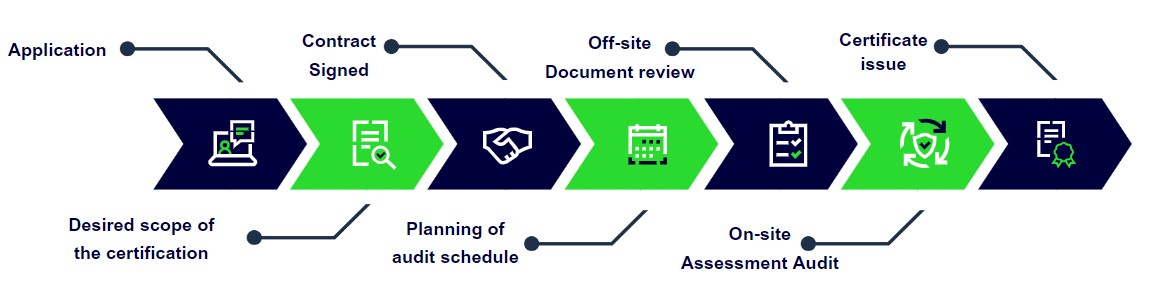
Certification Process